Fighting the Invisible Enemy with Vaccines Beginning of the End of the COVID-19 Pandemic
Download
Authors: Avanti A Srinivasan (1st-year Biology Honors College Student, New Jersey, USA); Keerthika Gnanasegaran (MBBS, Puducherry, India); Vishu Priya (MBBS, Puducherry, India).
Keywords:
COVID, Coronavirus disease; COVID-19, SARS-CoV-2, Severe Acute Respiratory Syndrome Corona Virus 2 identified in 2019 that causes COVID; Flattening the curve, The longer it takes for the coronavirus to spread through the population, the more time the health care systems (hospitals) have to prepare and treat patients, but not be overwhelmed by the pandemic; Herd immunity, when most of a population (70-80%) becomes immune to COVID-19, they provide indirect protection to those who are not immune to the disease; Immunity, protection from an infectious disease. If a person is immune to COVID that person can be exposed to the virus without becoming infected; Immunization, the process by which a person becomes protected against an infectious disease by vaccination; Vaccine, a biological preparation or substance (also known as antigen) that is used to stimulate the production of antibodies and provide immunity against COVID-19, without inducing the disease. Vaccines are usually administered by needle injections; Vaccination, the process of introducing a vaccine (prepared from the COVID, its products such as protein or DNA, or a synthetic substitute such as mRNA) to act as an antigen, into the body to induce immunity against COVID-19 disease.
COVID-19 Pandemic
Corona Virus disease (COVID-19) was first identified in Wuhan, China, in December 2019. It is caused by Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2). The highly contagious coronavirus has spread rapidly around the world exponentially, causing a pandemic.
As of June 04, 2021, there were over 172,231,339 confirmed cases and 3,703,522 deaths globally according to Johns Hopkins University (JHU) COVID-19 Dashboard. The USA alone has accounted for over 33,327,112 confirmed cases with over 596,444 fatalities reflecting the heavy toll inflicted by the pandemic. India has reported over 28,574,350 confirmed cases and 340,702 deaths, which is likely a low estimate.
The US Government at the beginning of the pandemic embarked on the “Operation Warp Speed” program to accelerate testing, supply, development, and distribution of safe and effective vaccines, therapeutics, and diagnostics for COVID-19 by January 2021. The program has led to the development of several effective vaccines against COVID-19 by commercial enterprises. The new US administration has focused all its efforts to vaccinate the US population starting January 2021 with the goal of reaching herd immunity by July 4, 2021; it is on its way to successful completion by the target date.
Initially, the number of confirmed COVID-19 cases reported by India was less, which was probably due to the quick action taken by the Indian Government to implement a total lockdown of the country to control the community spread of the virus. This was a very successful strategy for the short term and helped to flatten the curve and slowed the infection rate (Fig. 1). However, without an effective vaccine, with the Indian economy stagnating, with a population of 1.3 billion most of whom are poor, and a highly mutating virus, mitigation efforts alone proved to be grossly inadequate over the long term.
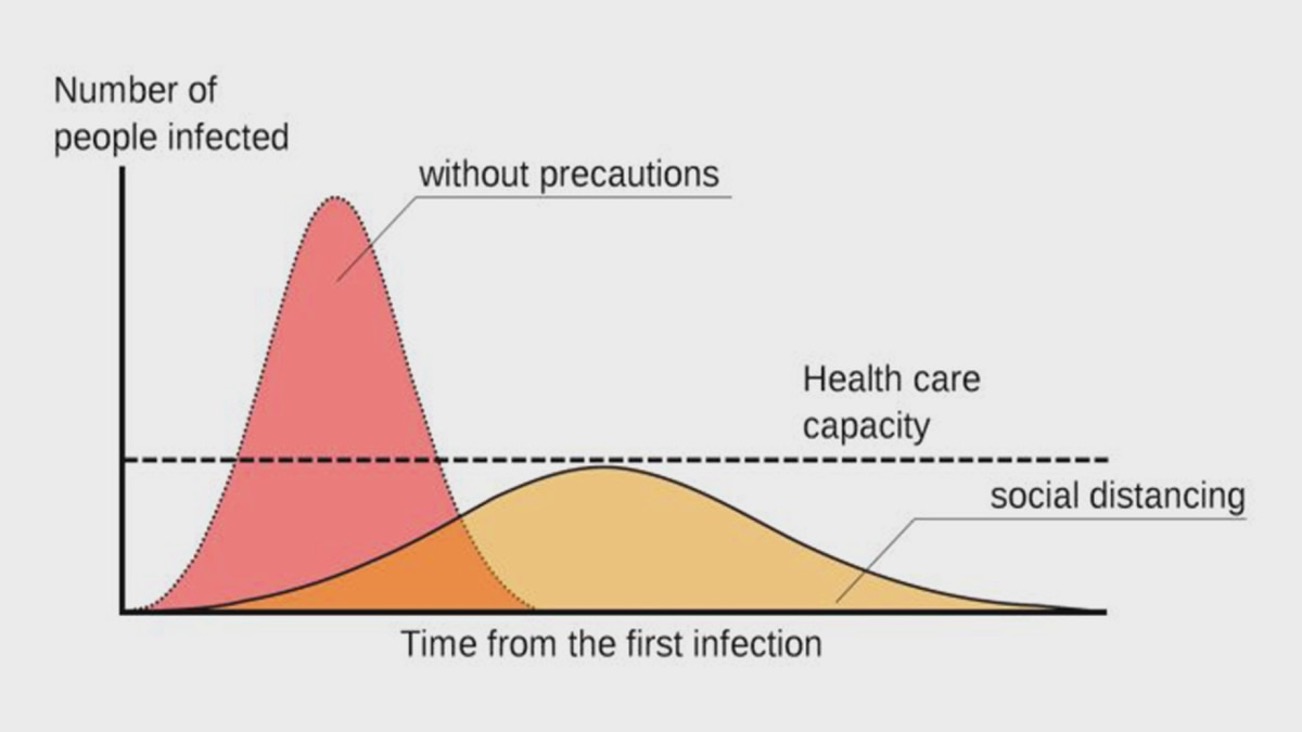
Fig 1: USA Center for Disease Control (CDC) graphic on flattening the curve
Over the past two months, India’s coronavirus daily infections have averaged over 400,000 and 4,000 deaths. They have come down recently, averaging daily infections of less than 200,000 and 2,800 deaths. Vaccinating the Indian population and reaching herd immunity, may be the only option left to fight the invisible enemy and to successfully put an end to the pandemic. India so far has inoculated only about 3% of its 1.3 billion people. India has a long way to go to get 70-80% of its population vaccinated and reach herd immunity.
What is immunity?
Humans are constantly exposed to disease-causing pathogens such as viruses, bacteria, fungi, and parasitic worms. Our body has two lines of defence against these threats: innate immunity and adaptive immunity, which together constitute the immune system. Their collective defence against pathogens makes up the immune response. The two components of the immune system interconnect and communicate at chemical and cellular levels to provide powerful protection against pathogens.
Innate immunity provides an immediate, nonspecific response against any invading pathogen and has no memory of prior exposure to the pathogen. Innate immunity relies on the recognition of certain foreign molecules to stimulate inflammatory responses and phagocytosis. Innate immunity is the first line of defence against pathogens, representing a critical systemic response to prevent infection and maintain homeostasis. It also contributes to the activation of an adaptive immune response. It does not adapt to a specific external stimulus or a prior infection but relies on genetically encoded recognition of molecular patterns.
The innate immune system recognizes pathogen-associated molecular patterns that are associated with pathogenic organisms but are absent in the host. The patterns are recognized by pattern recognition receptors of phagocytic cells such as toll-like receptor that are found on the cell surface and within the cell on various membrane-bound compartments. Cell surface receptors on macrophages (white blood cells) recognize and bind to surface molecules on the pathogen, activating the macrophage to phagocytize (engulf) the pathogens. Activated macrophages secrete cytokines, which bind to receptors on other host cells to trigger a successful immune response.
Adaptive (or acquired) immunity is specific; it recognizes individual pathogens and mounts an attack that directly neutralizes or eliminates them and retains a cellular memory of a pathogen; it reacts quickly upon second exposure to the same pathogen. The innate immune system provides some immediate protection against invading pathogens while the more powerful, specific, adaptive response system is mobilized that can take several days. Adaptive immunity, also known as acquired immunity, is a host immune response that is mediated by antigen-specific lymphocytes. Unlike innate immunity, the acquired immunity is highly specific to a particular pathogen, including the development of immunological memory. Like the innate system, the acquired system includes both humoral immunity components and cell-mediated immunity components. T cells differentiate from stem cells in the bone marrow and are carried in the blood to the thymus to generate two types of T cells (helper T cells and cytotoxic T cells) that are involved in adaptive immunity. Humoral immunity arises from B cells that differentiate from stem cells in the bone marrow and are carried in the blood to capillary beds serving the tissues and organs of the lymphatic system. In antibody (humoral)-mediated immunity, B-cell derivatives called plasma cells to secrete antibodies – highly specific protein molecules – that circulate in the blood and lymph recognizing and binding to antigens and clearing them from the body. In cell-mediated immunity, a particular type of T cell becomes activated and, in conjunction with other cells of the immune system, attacks foreign cells directly and kills them. Specific receptors on the plasma membrane of one B cell or T cell (B-cell receptors or T-cell receptors) bind to one specific antigen structure, also known as epitopes (Fig. 2).
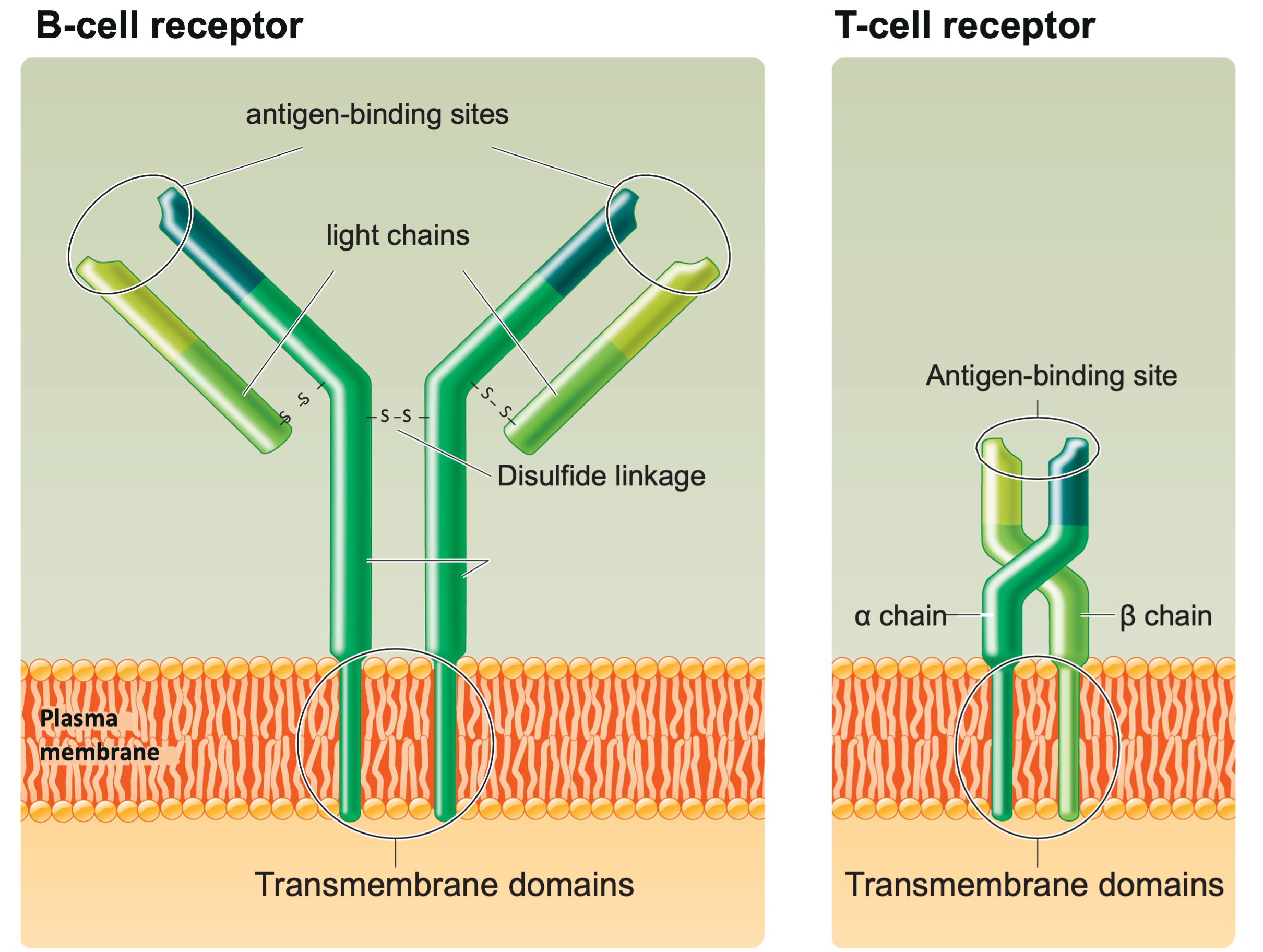
Fig. 2: Structure of B-cell and T-cell receptors
The adaptive immune response includes four key steps: 1) Antigen encounter and recognition: lymphocytes encounter and recognize an antigen; 2) Lymphocyte activation: lymphocytes are activated by binding to the antigen and divide to produce clones; 3) Antigen clearance: large clones of activated lymphocytes clear the antigen from the body; and 4) Development of immunological memory: memory cells circulate in blood and lymph, prepared for a rapid response in a future encounter of the same pathogen. The entire population of B cells and T cells in the body includes about 100 million different kinds of receptors – enough of a repertoire to recognize and destroy any type of antigen. Importantly, these cells are present even before the body has encountered the antigens.
Adaptive immunity can be acquired either naturally by infection or by vaccination. Adaptive immunity can be active or passive. Active immunity is acquired through exposure to a pathogen, which triggers the production of antibodies by the immune system. Passive immunity is acquired through the transfer of antibodies or activated T cells derived from an immune host either artificially or through the placenta from the mother.
What is a vaccine?
In 1796, Edward Jenner infected healthy individuals with cowpox, which prompted their immune systems to protect them against smallpox, a more deadly viral disease. Jenner’s technique became the basis for worldwide vaccination against smallpox, which now has been eradicated from the human population. This forms the basis for vaccination against other deadly pathogens.
An antigen is a foreign molecule that triggers an adaptive immune response. A vaccine is usually made from weakened or killed forms of the microbe, or typically contains an antigen from the disease-causing microorganism such as its toxins, or one of its surface proteins. The antigen stimulates the body’s immune system to recognize it as a threat and destroy it and to further recognize and destroy those microorganisms in a future encounter. Vaccines can be prophylactic (to prevent or ameliorate the effects of a future infection by a pathogen), or therapeutic to fight a disease that has already occurred. The administration of vaccines is called vaccination or inoculation. Vaccination is the most effective way to prevent infectious diseases. Widespread immunity due to vaccination is largely responsible for the worldwide eradication of smallpox and the restriction of diseases such as polio, measles, and tetanus.
Molecular structure of SARS-CoV-2
SARS-CoV-2 is a large, enveloped, spherical virus that contains a positive-sense, single-stranded RNA genome (30 kb in size), which is packed inside the nucleocapsid protein (N) and surrounded by an envelope. The RNA genome has a 5′ capped structure and a 3′ poly-A tail. The 5′ terminal two-thirds of the genome encodes a polyprotein, pp1ab, which is further cleaved into 16 non-structural proteins that are involved in genome transcription and replication. The 3′ terminus encodes 3 different structural proteins.
Membrane proteins (M) and envelope proteins (E) are involved in virus assembly. The M protein (~30 KDa) is the most abundant structural protein in the virion. The E protein (~12 KDa) is found in small quantities within the virion.
Spike protein (S1) that mediates virus entry into host cells, is the target of all COVID-19 vaccines. The spike protein forms large protrusions from the virus surface, giving it the appearance of having crowns (Fig. 3). Spike protein contains an S1 subunit that is a Receptor Binding Domain (RBD) and a membrane-fusing spike S2 subunit; The entry receptor utilized by SARS-CoV-2 is Angiotensin Converting Enzyme II (ACE II). Upon binding S1 is processed into S2, which induces fusion of the host and viral membranes.
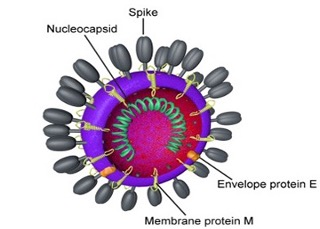
Fig. 3: Structure of the coronavirus, SARS-CoV-2 that causes COVID-19 [adapted from Centers for Disease Control (CDC) and Prevention, USA].
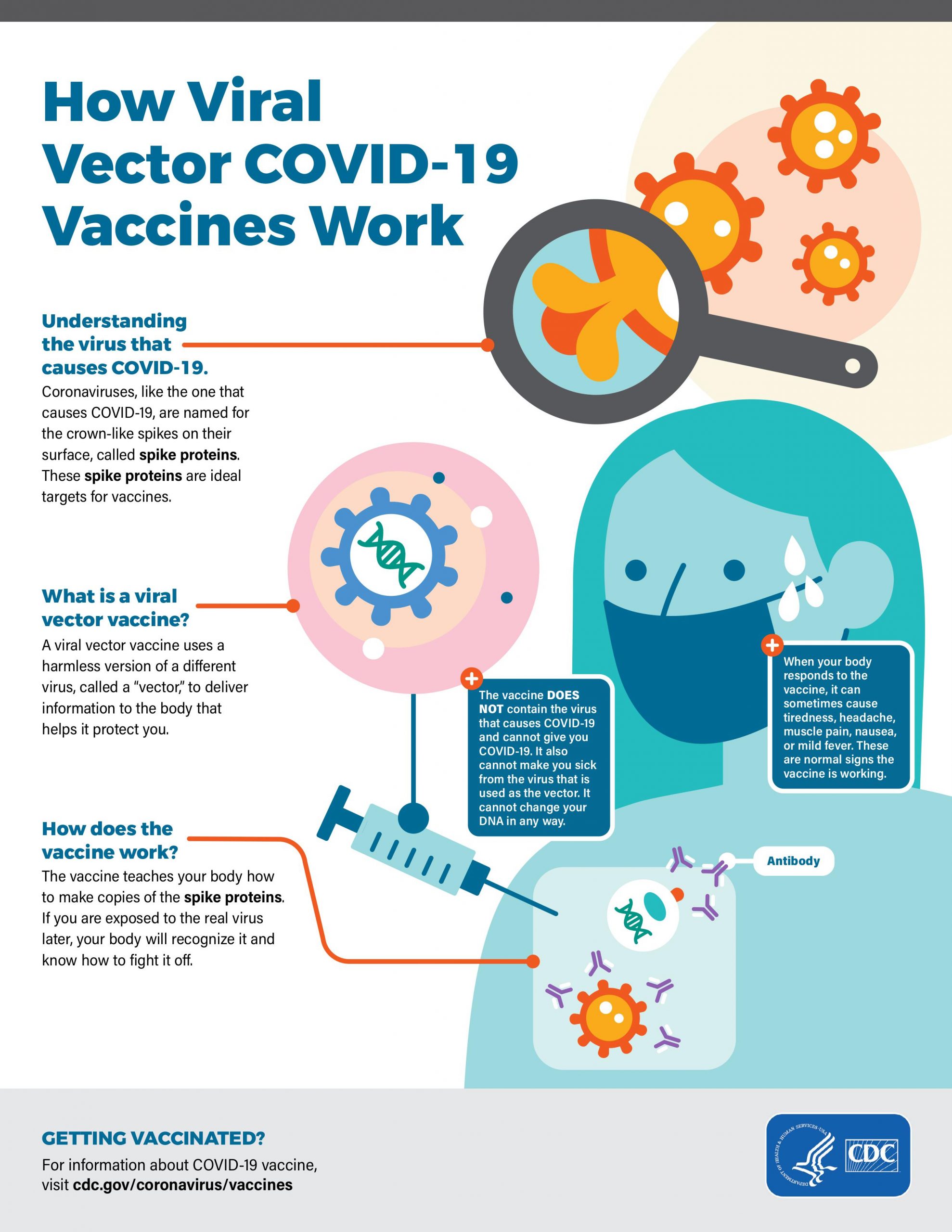
COVID-19 viral vector vaccines
Viral vector vaccines are a modified version of a different virus to deliver instructions to the cell to make the antigen against coronavirus spike protein. People vaccinated with viral vector vaccines gain protection without ever having to risk the serious consequences of getting sick with COVID-19. Several important characteristics of the viral vector need to be pointed out that include: 1) The vector is not the virus that causes COVID-19; it is a different harmless virus that is engineered to carry the gene coding for the spike protein, a harmless piece of the coronavirus. The modified version of the virus will be injected into the body and the cells will produce the spike protein that is found only on the surface of coronavirus. The cells display spike protein on their surface, triggering an immune response against the spike protein antigen. The immune cells produce antibodies and activate T cells to fight off the infection. The net result is the body has learned to recognize spike protein and to protect us against any future infection by the virus that causes COVID-19. The vaccine protects us, without ever having to risk the serious consequences of getting sick with COVID-19. Any temporary discomfort (side effects) experienced by us immediately after getting the vaccine is a natural part of the process and indicates that the vaccine is working to stimulate our immune system. Viral vector vaccines have been around for a while; they are safe and effective. COVID-19 viral vector vaccines were developed using adenoviral vector by two commercial entities, namely AstraZeneca and Johnson & Johnson (Appendix I).
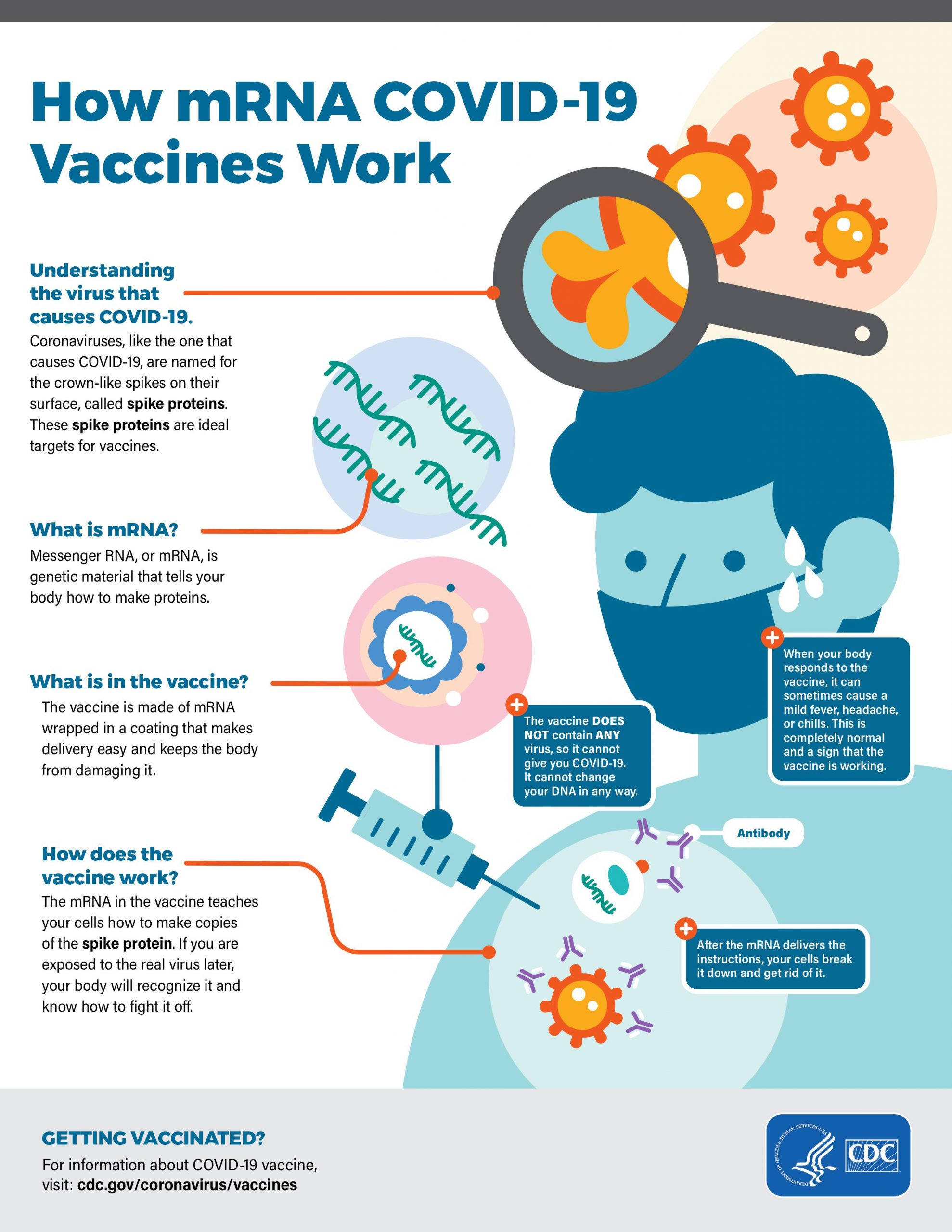
COVID-19 messenger RNA (mRNA) vaccines
mRNA vaccines are a new type of vaccine to protect against infectious diseases. To trigger an immune response, most vaccines inject a weakened or inactivated pathogen into our bodies. mRNA vaccines, on the other hand, teach our cells how to make a protein antigen (or even a piece of a protein) within cells to induce an immune response in our bodies. The focus of the mRNA COVID-19 vaccine is to teach cells how to make spike protein, and thereby, trigger an immune response in our bodies. Like the viral vector vaccines, people vaccinated with mRNA COVID-19 vaccines gain protection without ever having to risk the serious consequences of getting sick with COVID-19. The mRNA vaccines are some of the first COVID-19 vaccines that were authorized for emergency use by the US Government. mRNA vaccines can be developed easily in a laboratory using readily available materials. Furthermore, the process of making mRNA vaccines can be readily standardized and scaled up, making vaccine development much faster than the traditional methods of making vaccines. As soon as the genome sequence of the virus that causes COVID-19 became available, scientists began designing the mRNA instructions for cells to build the unique spike protein into an mRNA vaccine. Effective COVID vaccines became available in less than a year to vaccinate the US population from two commercial enterprises, namely Moderna and Pfizer-BioNTech (Appendix II). The US Government has been very successful in administering the COVID-19 vaccines to its population. The USA is on the verge of reaching herd immunity by July 4, 2021. The success of the vaccination effort can be seen from the fact that several states in the USA have started lifting all mandatory mitigation efforts including the mask mandate.
CDC (USA) Guidelines for Side Effects of COVID-19 Vaccines
COVID-19 vaccines are highly effective, but they are also “reactogenic”, meaning that they are likely to cause a noticeable immune response or side effects. Side effects may vary with the type of COVID-19 vaccine. The most common side effects include soreness at the site of injection, fatigue, headache, muscle aches, chills, joint pain, and fever (Table 1). The side effects usually last 24 to 48 hours, and no more than a few days. Side effects were more frequent after the second dose in the vaccine trials. These side effects are typical of the inflammation induced by vaccines and are a sign of the body’s immune response to the vaccine. Some people have more severe reactions than others. Side effects have been less frequent and less severe in adults older than 55 years in the vaccine trials.
The first dose by itself will not provide complete protection, and it will take about seven days after the second dose before one achieves a full protective level of immunity that develops in about 95% of vaccine recipients. If one is exposed to SARS-CoV-2 before this time, it is possible that the person could develop COVID-19. Even once a person has received both doses of the COVID-19 vaccine, it will still be important to continue practising public health mitigation strategies like masks and social distancing until the pandemic is under control and till we know more about how the vaccines prevent transmission. The side effects of the vaccine typically start within 12 to 24 hours of vaccination. If you experience side effects that last beyond 48 hours, you should contact your doctor or medical provider for advice.
COVID-19 vaccination will help to protect you from getting COVID-19. You may have some side effects, which are normal signs that your body is building protection. Side effects may affect your ability to do daily activities, but they should go away in a few days.
Common side effects:
On the arm where you got the shot:
- Pain
- Swelling
Throughout the rest of your body:
- Fever
- Chills
- Fatigue & tiredness
- Headache
Helpful tips:
If you have pain or discomfort, talk to your doctor about taking over-the-counter medications, such as ibuprofen or acetaminophen.
To reduce pain and discomfort where you got the shot:
- Apply a clean, cool, wet washcloth over the area.
- Use or exercise your arm.
To reduce discomfort from fever:
- Drink plenty of fluids.
- Dress lightly.
When to contact a doctor:
In most cases, discomfort from fever or pain is normal. Contact your doctor or healthcare provider:
- If the redness or tenderness where you got the shot increases after 24 hours
- If your side effects are worrying you or do not seem to be going away after a few days.
Some things to remember:
- Side effects may feel like flu and even affect your ability to do daily activities, but they should go away in a few days.
- With most COVID-19 vaccines, you will need 2 shots for them to work effectively. Get the second shot even if you have side effects after the first shot unless a vaccination provider or your doctor tells you not to get a second shot.
- It takes time for your body to build protection after any vaccination. COVID-19 vaccines that require 2 shots may not protect you until a week or two after your second shot.
It is important for everyone to continue using all the tools available to help stop this pandemic as we learn more about how COVID-19 vaccines work in real-world conditions. Cover your mouth and nose with a mask when around others, stay at least 6 feet away from others, avoid crowds, and wash your hands often.
SARS-CoV-2 variants
Viruses are constantly mutating and changing, that includes SARS-CoV-2, the virus that causes COVID-19. These genetic variations occur over time and can lead to the emergence of new variants that may have different properties. The SARS-CoV-2 genome encodes instructions organized as genes, to build the virus. Genomic sequencing allows scientists to identify SARS-CoV-2 and monitor how it changes over time into new variants, understand how these changes affect the characteristics of the virus, and use this information to better understand how it might impact health.
It is important to monitor circulating viruses for key mutations that happen in important regions of the genome like the gene coding for spike protein. For instance, variants of the spike protein gene sequence can alter the amino acid sequence of the spike protein, which could alter the effectiveness of the antibody treatment and the immunity developed through vaccination. Many mutations do not affect the virus’s ability to spread or cause disease because they do not alter the major proteins involved in infection; eventually, these are outcompeted by variants with mutations that are more beneficial for the virus.
As per CDC (USA), surveillance of emerging variants can help detect coronavirus variants with:
- Ability to spread more quickly in people.
- Ability to cause either milder or more severe disease in people.
- Ability to evade detection by specific diagnostic tests.
- Decreased susceptibility to medical therapies that employ monoclonal antibodies. (Such therapy involves specifically designed antibodies that target regions of the virus to block infection. Because these treatments are more specific than natural immune response-generated antibodies, they may be less effective against variants that emerge).
- Ability to evade natural or vaccine-induced immunity (Both natural infection with and vaccination against SARS-CoV-2 produces a polyclonal antibody response that targets several parts of the spike protein. The virus would need to accumulate significant mutations in the spike protein to evade immunity induced by vaccines or by natural infection).
Among these, the ability to evade vaccine-induced immunity would be the most concerning. Several coronavirus variants have evolved mutations to spread more easily, make people sicker, escape immune responses, evade tests, or render treatments ineffective. These are called “variants of concern” by WHO. There are four coronavirus variants that experts around the world are particularly worried about. These variants were first identified in South Africa, the UK, Brazil, and India respectively (Table 2).
COVID-19 variants of concern
1) B.1.1.7, first found in the UK (WHO name: Alpha)
B.1.1.7 was first detected in two people in South-East England. It has been identified in 123 countries worldwide, including the US. It became the most common variant in the US. Tennessee has the highest proportion of B.1.1.7 cases of any state, accounting for 73% of sequenced cases. B.1.1.7 is between 30% to 50% better at spreading from person to person than other coronavirus variants, according to UK scientists. B.1.1.7 could be more deadly. However, two studies published in the Lancet Infectious Diseases and the Lancet Public Health indicated that B.1.1.7 was more infectious, but didn’t cause worse illness in hospitalized patients. COVID-19 vaccines from Pfizer-BioNTech, Moderna, Jonson & Johnson and AstraZeneca all provide protection against B.1.1.7. all provide protection against B.1.1.7.
2) B.1.351, first identified in South Africa (WHO name: Beta)
B.1.351 was first detected in South Africa, in samples dating back to the beginning of October 2020. It has been found in 84 countries, including the US. B.1.351 is thought to be 50% more contagious than the original strain. Data suggests that the variant may evade the body’s immune response. Antibodies work best when they bind well to the virus and stop it from entering our cells. The B.1.351 variant has mutations called E484K and K417N at the site where antibodies bind. In lab tests, antibodies produced by Pfizer and Moderna’s COVID-19 vaccines could not bind well to B.1.351, compared to the original coronavirus. In a real-world study, Pfizer’s vaccine was 75% effective at preventing infection of varying severity caused by the variant first found in South Africa, called B.1.351, after two doses. Johnson & Johnson COVID-19 vaccine was 64% effective at preventing COVID-19 in trials in South Africa, where 95% infections are caused by B.1.351, and 72% effective in the US, where B.1.351 accounted for less than 1% of sequenced coronavirus tests. This suggests that vaccines will not become completely useless against variants. Existing vaccines could be updated and tailored to a new variant within weeks or months, or you may require a booster shot.
3) P.1, first identified in Brazil (WHO name: Gamma)
The variant found in Brazil was first detected in four people in Japan, who had travelled from Brazil on January 2,. It has been found in 45 countries worldwide, including the US. P.1 is twice as contagious as the original coronavirus. P.1 has similar E484K and K417T mutations as B.1.351, which means it can evade antibody responses. This could be the reason P.1 reinfects people who have already caught coronavirus. A recent study published on April 14 showed that previous coronavirus infection only offered between 54% and 79% of the protection for P.1 than for other virus strains. P.1’s mutations could also mean that vaccines work less well. COVID-19 vaccines from Pfizer and AstraZeneca work against P.1. Johnson & Johnson’s COVID-19 vaccine was 68% effective in trials in Brazil, where the variant is the most common strain, compared with its 72% efficacy in the US, where P.1 at the time accounted for 0.1% of sequenced coronavirus tests.
4) B.1.617, first identified in India (WHO name: Delta)
The variant first found in India, B.1.617, is in fact three distinct viruses. Collectively, they have spread to more than 17 countries. All three have been detected in the US. The WHO and UK have designated it a “variant of concern” because it is more infectious than the original virus. The mutations include: L452R, may make the virus more infectious or it may avoid the antibody response; P6814, may make it more infectious; and E848Q, may help the virus avoid the antibody response. Health officials in England recently reported that two doses of the COVID-19 vaccines made by Pfizer-BioNTech or AstraZeneca are highly protective against variants first detected in India and the United Kingdom. The data also underscored the need for two doses, as both vaccines were significantly less effective after only one shot. The vaccines were similarly effective at protecting against the UK variant. Moderna vaccine also appears to protect against COVID variants, B.1.617 and B.1.618 that were first identified in India.
Moderna reported that its COVID-19 vaccine was 100% effective in a trial involving 3732 adolescents aged 12-17, with no major safety concerns. Among adolescents who received two doses, there were no cases of COVID-19 compared with four cases among those who received a placebo. After only one dose, the vaccine was 93% effective in the age group. Side effects were similar to first reported in earlier studies, including headache, fatigue, body ache, fever and chills. Rare cases of a few adolescents and older teenagers developing myocarditis (mild heart problems) after receiving the COVID-19 vaccines was reported. CDC is investigating whether this is a possible side effect of vaccination or if they are merely a coincidence. The relatively few cases seem to have occurred approximately four days after the second dose of mRNA vaccines made by either Pfizer-BioNTech or Moderna. Symptoms have been more common in males than females. Some rare cases of females developing blood clots after receiving the AstraZeneca vaccine has also been reported. Moreover, it appears that COVID-19 survivors with lingering symptoms can safely be vaccinated against the coronavirus.
More recently, WHO has named the four variants of concern, known as the UK (B.1.1.7), South Africa (B.1.351), Brazil (P.1) and India (B.1.617.2) with Greek alphabets Alpha, Beta, Gamma, and Delta, respectively.
“Breakthrough” infections after vaccinations
Based on roughly 101 million Americans fully vaccinated against COVID-19, CDC reported that breakthrough infections occurred in 0.01% of them. Approximately, 27% of breakthrough infections were asymptomatic, while in 2% of the cases, patients died. The CDC sequence data for virus samples from 555 breakthrough infections indicated that mutated variants of the coronavirus, those were first seen in the UK and South Africa, accounted for 64% of the breakthroughs. Moderna and Pfizer are developing booster shots to combat COVID-19 variants.
COVID-19 disease & black fungal infection
A rare and potentially deadly infection by mucormycosis (also known as black fungus), has been observed in several coronavirus patients, or those who have recently recovered from COVID-19, whose immune systems have been weakened by the virus or who have underlying conditions, most notably diabetes. Over 6000 black fungus cases have been reported across India, with hundreds hospitalized and at least 100 dead.
Black fungus is caused by mould found in damp environments (like soil or compost) and can attack the respiratory tract. It is not contagious and does not spread from person to person. Black fungus commonly affects the sinuses or lungs after a person inhales fungal spores in the air and can also affect the skin following a surface injury like a cut or burn. Symptoms depend on where in the body the fungus is growing but can include facial swelling, fever, skin ulcers and black lesions in the mouth. Black fungus disease begins to manifest as skin infection in the air pockets located behind our forehead, nose, cheekbones, and in between the eyes and teeth. It can then spread to the eyes, lungs and can even spread to the brain. It leads to blackening or discolouration over the nose, blurred or double vision, chest pain, breathing difficulties and coughing of blood. If it is not controlled or treated, the mortality rate could be from 20% to 50%. The mortality rate also depends on which part of the body is affected; it is less deadly for people with sinus infections but more deadly for those with lung infections.
Immunocompromised people are more susceptible to infection who include COVID-19 patients, diabetic patients, people who take steroids, and those with other comorbidities like cancer or organ transplants. COVID-19 patients are particularly susceptible because not only does the virus affect their immune system, drugs used to treat the disease can also suppress their immune response. Due to these factors, COVID-19 patients face a renewed risk of failing the battle against attacks mounted by the black fungus. This does not mean that every COVID-19 patient will get infected by the black fungus as it is uncommon among those without diabetes. The prevalence of diabetes in India is as high as 12% to 18% of the adult population, especially in urban areas.
Black fungus is treated with antifungal medicines such as Amphotericin B that is given intravenously. Patients may need up to six weeks of anti-fungal medicine to recover. Their recovery depends on how early the disease was diagnosed and treated. Often, surgery is required to cut away dead or infected tissue. For some patients, this may mean loss of the upper jaw or sometimes even the eye. Black fungus is 70 times more prevalent in India, possibly due to several factors that include: 1) higher rate of “undiagnosed” and “uncontrolled” diabetes; 2) tropical humid climate that promotes fungal growth; and 3) delays in seeking medical attention and diagnosing the disease, and challenges in managing the advanced stage of infection. COVID-19 pandemic has worsened the situation in India, by promoting opportunistic infection by the black fungus.
Authors personal experience with COVID-19 vaccine side effects
Avanti Srinivasan (1st-year Biology Honors College Student and working a part-time summer job at Penn Medicine Princeton Health): It is evident that COVID-19 has turned the world upside down. After almost a year of quarantine, death and chaos, the pandemic has also now opened a new era in vaccine development with new technologies. As a college student, when I heard about the vaccine I was delighted as I was ready to return to normalcy and resume my life where I left off one year ago after finishing my senior year of high school. I received the Pfizer-BioNTech COVID-19 vaccine on April 18th, 2021. I was quite nervous before receiving the shot as my friends had warned me about various side effects they felt from the vaccine after getting their first dose. Luckily, unlike many others, I did not feel any side effects from the vaccine. One day after vaccination, I felt a slight pain at the injection site, but this is a common immune response to receiving any vaccine as it shows that our immune system is working properly. Three weeks later, I received my second dose of Pfizer-BioNTech COVID-19 vaccine on May 9th, 2021. Just like the first dose, I did not feel any harsh side effects. The usual pain near the injection site and tiredness were there, but it got better after 2-3 days. Overall, I would encourage everyone to get vaccinated as soon as possible. After receiving both doses of the vaccine, I feel more confident and protected and have resumed normal activities without fear of the coronavirus. I know that even if I do get infected with coronavirus, I will not become seriously ill, as the vaccine will provide me with a layer of protection from the deadly virus. After getting vaccinated I also feel that I am playing my role as a good citizen and community member in my state by helping to prevent the spread of COVID-19. I am also encouraging those around me to get vaccinated and by doing so, we will reach herd immunity at which point we can finally put the pandemic behind us and move forward with our lives.
Dr Keerthika Gnanasegaran (currently working in a multi-speciality hospital in Pondicherry and an INICET aspirant): I got vaccinated with COVISHIELD at my hospital on March 10, 2021. At first, I was very scared about getting the COVID-19 vaccine. I surfed many websites and got advice from many health care professionals, which convinced me to change my mind about getting vaccinated. One day after vaccination of the first dose, I got injection site pain, severe headache, fever >102⁰ F, and fatigue. I consulted my Chief at the Hospital and he said not to worry and advised me to take a Paracetamol tablet once every 6 hours. The following day, I felt alright except for some mild injection site tenderness. Unfortunately, just before I was about to take my second dose of inoculation, I tested positive for COVID-19. I did not have any symptoms except mild body ache. My father, who is obese, diabetic, and suffers from hypertension, also tested positive for COVID-19 after the first dose of vaccination. He also had only mild body ache and we both were under home isolation. Finally, I realized, getting COVID-19 vaccination very likely prevented us from a serious illness. I plan to get my second dose of COVID-19 vaccination after six weeks. Based on my personal experience, I request and encourage everyone to go ahead, shed their inhibition about getting COVID-19 vaccination.
Dr Vishnu Priyaa Radjassegarane (a medical student doing her postgraduate studies in Pediatrics in Pondicherry): As a medical student, I came to know about the seriousness of the COVID-19 disease at the hospital. During the early phase of the COVID-19 pandemic, I became infected with the virus, and thankfully I recovered from the infection after treatment. Initially, like many others, I had many doubts regarding the COVID-19 vaccine and was afraid of its side effects. But upon reflecting some more, I decided to get the COVID-19 vaccination done. I reasoned that even if I get reinfected again with the coronavirus, I could avoid a severe illness like getting admitted to ICU or being on a ventilator. I got my first dose of COVISHIELD inoculation on March 15, 2021. With the information that I gathered from my fellow postgraduates regarding the side effects of the vaccine, I took my pain killers prophylactically even before the symptoms could appear. After vaccination, I had some side effects: low-grade fever, injection site pain, myalgia and difficulty in lifting my arm. But these lasted for only 2 days and then subsided. After 6 weeks, I got my second dose of the COVISHIELD vaccine on April 24, 2021. But luckily, I did not experience any side effects after the second dose like the first. After getting the two doses of the COVID-19 vaccine, I feel very confident and safe to go back to work at my hospital, even looking after patients with COVID-19. I will never say that I will not be reinfected with the coronavirus again, but with the COVID-19 vaccination, I feel that I will not get a severe form of the COVID-19 disease that requires oxygen supply or ventilator support. In my opinion, I believe that most of the Pondicherry and Tamil Nadu citizens and the Indian population should be vaccinated as soon as possible to reach herd immunity when we can together put an end to this deadly pandemic and return to our normal life and walk outside confidently without masks.
Summary
COVID-19 vaccines offer the best way to fight the invisible enemy and overcome the COVID-19 pandemic. US President Biden has focused all his efforts to get at least 65-70% of the US population vaccinated in the first 180 days of his administration with at least one dose to reach herd immunity. The US is well on its way to successfully achieve this goal by July 4, 2021. Vaccinating the Indian population and reaching herd immunity, may be the only option left for the Indian Government to fight the invisible enemy and to successfully end the deadly COVID-19 pandemic. India so far has inoculated only about 3% of its 1.3 billion people, has a long way to vaccinate 70-80% of its population to reach herd immunity. COVID-19 variants could pose a problem by reducing the effectiveness of the vaccines. This could be addressed by giving booster shots against new COVID-19 variants.
Acknowledgement
This article was put together using the information from the Center for Disease Control and Prevention (CDC, USA), World Health Organization (WHO), CNN and from various news articles (too numerous to list them all here). As a disclaimer, we must emphasize that this article is meant to serve solely as an informational resource for the readership. People affected by the coronavirus should consult with their physician for advice and treatment as well as for information about COVID-19 vaccination.
Table 1: COVID-19 vaccines and their side effects
| Vaccine | Status | Dosing | Efficacy | Potential Side Effects |
| Pfizer | Vaccine has been authorized for emergency use | Two doses, delivered three weeks apart | 95% effective at preventing serious illness | Injection site pain, fatigue,
headaches, chills |
| Moderna | Vaccine has been authorized for emergency use | Two doses, delivered four weeks apart | 94% effective at preventing serious illness | Injection site pain, fatigue,
muscle aches, joint pain, headaches, chills |
| Johnson & Johnson | Awaiting emergency use authorization by the FDA | One Dose | 72% effective at preventing severe illness | Injection site pain, fatigue,
headache, muscle pain, joint pain |
| Novavax | Vaccine trials are ongoing | Two doses, delivered three weeks apart | – | Injection site pain, rash,
headaches, muscle pain, fever |
| Covishield (AstraZeneca/Serum Institute of India) | Central Drug Standard Control Organization (CDSCO) India granted Emergency Use Authorization (EUA) | Two doses, delivered 12 to 16 weeks apart | 63% effectiveLonger dose intervals with 12 weeks range associated with greater vaccine efficacy | Injection site pain, headache,
fatigue, myalgia, fever rarely one-sided facial nerve palsies, demyelinating disorders |
| Covaxin (Bharat Biotech) | Central Drug Standard Control Organization (CDSCO) India granted Emergency Use Authorization (EUA) | Two doses, delivered four to six weeks apart | 78% effective in preventing serious illness | Injection site pain, headache,
fatigue, myalgia, fever, body ache, tremors, giddiness, cold, cough |
| Table 2: SARS-CoV-2 variants | |||
|
Name
|
Spike Protein Substitutions | First Detected | |
| B.1.525 |
Spike: A67V, 69del, 70del, 144del, E484K, D614G, Q677H, F888L |
United Kingdom/Nigeria – December 2020 |
|
| B.1.526 |
Spike: (L5F*), T95I, D253G, (S477N*), (E484K*), D614G, (A701V*) |
United States (New York) – November 2020 |
|
| B.1.526.1 |
Spike: D80G, 144del, F157S, L452R, D614G, (T791I*), (T859N*), D950H |
United States (New York) – October 2020 |
|
|
B.1.617 |
Spike: L452R, E484Q, D614G |
India – February 2021 |
|
| B.1.617.1 |
Spike: (T95I), G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H |
India – December 2020 |
|
| B.1.617.2 |
Spike: T19R, (G142D), 156del, 157del, R158G, L452R, T478K, D614G, P681R, D950N |
India – December 2020 |
|
| B.1.617.3 |
Spike: T19R, G142D, L452R, E484Q, D614G, P681R, D950N |
India – October 2020 |
|
|
P.2 |
Spike: E484K, (F565L*), D614G, V1176F |
Brazil – April 2020 |
|
(*) = detected in some sequences but not all
Appendix I
Appendix II
Editors’ comments
While it is difficult for any government to be fully prepared for a pandemic that occurs once every hundred years, it is important for a task force to review and put policies in place for future preparedness to deal with such a deadly pandemic. While India had successfully contained the coronavirus infection rate in the short term by mitigation efforts and complete lockdown, it failed to prepare for all possible contingencies, such as the emergence of a deadlier and more contagious COVID-19 variant. Furthermore, failure to curtail large gatherings for religious festivals and election-related activities may also have contributed to the rapid spread of the virus all over the Indian subcontinent. Consequently, the rate of infection soared; the Indian health care system was overwhelmed, leading to increased Indian mortality and morbidity. In hindsight, the Indian Government should have mobilized to vaccinate its population as soon as effective vaccines became available in early January 2021, to reach herd immunity and to make the Indian population immune to COVID-19. Overseas export of vaccines should have been curtailed immediately as the US Government did unilaterally with the export of raw materials (supply chains) needed to make the vaccines. India, though late and after catastrophic deaths, has ramped up its vaccination program. Vaccine shortages are gradually being addressed. Currently, 20 crores (200 million) people have been vaccinated at least with one shot, which is second only to the US. In terms of percentages, it is low because of the huge Indian population. The Indian government is increasing its efforts to ramp up vaccination to 30 crores (300 million) people a month by August 2021.
One bright spot that has emerged out of the misery of the pandemic is that it has helped us to recognize and laud the “real” heroes. They are many among us worldwide: doctors, nurses, first responders who risk their own lives to take care of the COVID-19 patients. The young authors of this article are representative of such real heroes worldwide.
Edited by S Chandrasegaran PhD and M Matheswaran PhD.
Dr S Chandrasegaran is Professor Emeritus at the Bloomberg School of Public Health, Johns Hopkins University, Baltimore, USA.
Air Marshal M Matheswaran (Retd) is the President of The Peninsula Foundation.